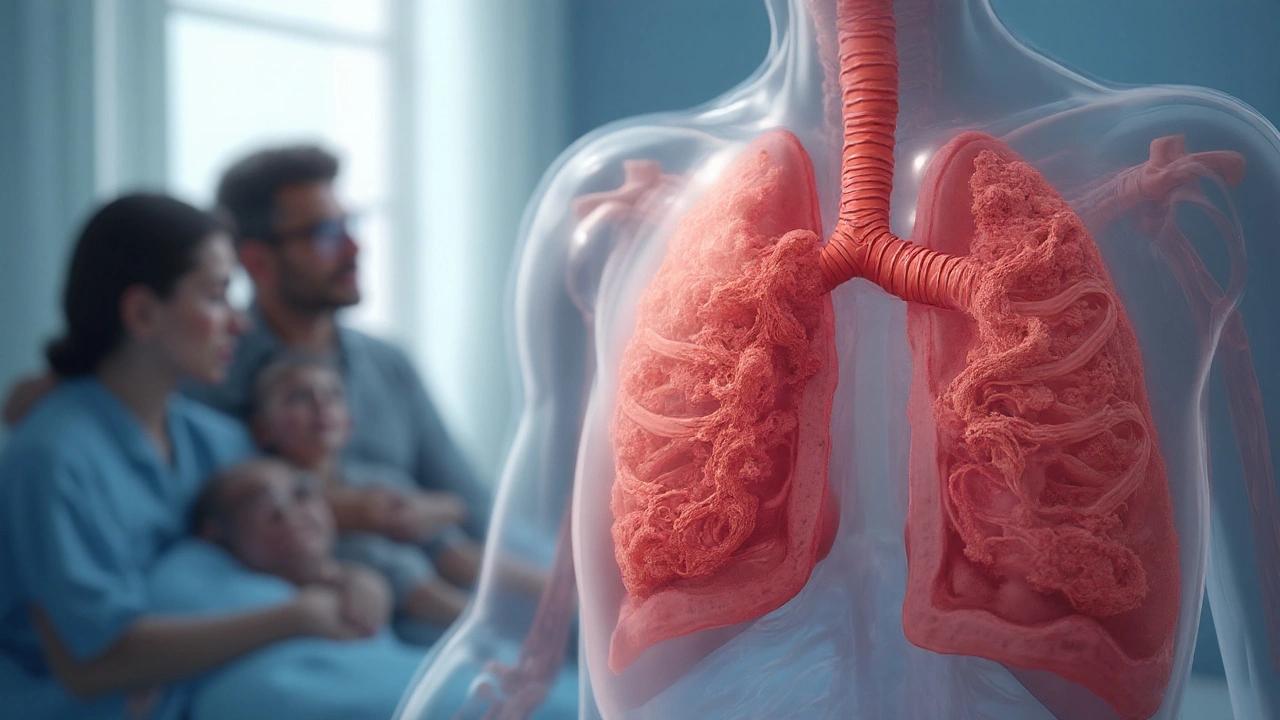
Cystic Fibrosis is a genetic disorder caused by mutations in the CFTR gene that leads to thick, sticky mucus in multiple organs, most notably the lungs. When that mucus builds up in the Respiratory System the network of airways, lungs, and breathing muscles that moves oxygen into the blood, it creates a perfect storm for infection, inflammation, and progressive loss of function.
What the CFTR Gene Does - and Why It Matters
The CFTR Gene codes for a chloride channel that regulates fluid balance on cell surfaces. In healthy people, this channel keeps airway surface liquid thin, allowing cilia to sweep mucus out. In cystic fibrosis, the defective channel traps chloride, pulling water into the mucus and making it viscous.
More than 2,000 CFTR mutations exist, but the most common (ΔF508) accounts for about 70% of cases. The severity of lung disease often tracks with how much residual CFTR function remains.
Viscous Mucus: The Core Problem in the Lungs
Mucus a gel-like secretion that traps particles and pathogens in a healthy airway is watery enough to be cleared quickly. In cystic fibrosis, mucus becomes ten times more viscous, sticking to airway walls and creating pockets where bacteria can thrive.
This thick mucus triggers chronic airway inflammation. White blood cells flood the area, releasing enzymes that gradually erode airway walls, leading to bronchiectasis-a permanent widening and scarring of the bronchi.
Common Culprits: Chronic Lung Infections
The sticky environment favors certain opportunistic bacteria. Pseudomonas aeruginosa a gram‑negative bacterium that forms biofilms and resists many antibiotics is the most notorious, colonizing the lungs of over 80% of adults with cystic fibrosis.
Other frequent offenders include Staphylococcus aureus, Burkholderia cepacia complex, and non‑tuberculous mycobacteria. Each infection spikes inflammation, accelerates lung function loss, and often forces a pulmonary exacerbation-a sudden worsening of symptoms that may require hospitalization.
How Lung Function Declines
Physicians track disease progression with spirometry, especially forced expiratory volume in one second (FEV1). In cystic fibrosis, average FEV1 declines about 1-2% per year without aggressive therapy, and the rate accelerates after age 18.
Chest imaging (high‑resolution CT) reveals bronchiectasis, mucus plugging, and air trapping. Together, these findings explain why patients experience chronic cough, daily sputum production, and shortness of breath during even mild activity.
Diagnostic Tools: From Sweat Test to Genetic Screening
The gold‑standard diagnostic test is the Sweat Test which measures chloride concentration in sweat; values above 60mmol/L are diagnostic of cystic fibrosis. Newborn screening programs now combine immunoreactive trypsinogen (IRT) levels with DNA analysis to catch the disease before symptoms appear.
Genetic sequencing confirms the specific CFTR mutations, guiding personalized treatment decisions-especially the use of CFTR modulators.

Modern Therapies That Target the Underlying Defect
For the first few decades, care focused on airway clearance, antibiotics, and nutritional support. The breakthrough came with CFTR modulators, small molecules that improve the protein’s function.
| Treatment | Mechanism | Key Benefits | Common Side Effects |
|---|---|---|---|
| Ivacaftor (Kalydeco) | Potentiates gating mutations | ↑ FEV1 by 10‑15%; reduces sweat chloride | Headache, rash |
| Lumacaftor/Ivacaftor (Orkambi) | Corrects ΔF508 processing + potentiates | Slows FEV1 decline, improves BMI | Elevated liver enzymes, cough |
| Traditional Antibiotics & Airway Clearance | Eradicates infection, clears mucus | Reduces exacerbations, improves sputum clearance | Antibiotic resistance, ototoxicity (aminoglycosides) |
Since 2019, triple‑combination regimens (elexacaftor/tezacaftor/ivacaftor) have shown >15% improvement in FEV1 for people with at least one ΔF508 allele, translating into better exercise tolerance and quality of life.
Supporting Strategies: Airway Clearance and Nutrition
Even with modulators, thick mucus still needs physical removal. Chest Physiotherapy techniques like percussion, postural drainage, and vibration devices remain cornerstone therapies. High‑frequency chest wall oscillation (the “vest”) can halve sputum volume in a single session.
Nutrition is another vital piece: pancreatic insufficiency affects 85% of patients, requiring enzyme replacement and high‑calorie diets to support lung growth and immune function.
Living With a Respiratory‑Focused Disease
Daily life for a teenager with cystic fibrosis often means a morning routine of enzyme pills, inhaled bronchodilators, and a 30‑minute vest session before school. Social activities can be limited by infection control-many clinics advise against close contact with individuals carrying contagious viruses like RSV.
Psychological support is essential. Chronic disease burden contributes to anxiety and depression rates twice that of the general population. Integrated care teams that include psychologists, dietitians, and respiratory therapists improve adherence and outcomes.
Future Directions - What’s on the Horizon?
Gene therapy and mRNA approaches aim to deliver a functional CFTR copy directly to airway cells, potentially offering a cure. Early-phase trials using viral vectors have reported modest CFTR activity without major safety concerns.
CRISPR‑based editing is also being explored to correct the ΔF508 mutation in stem cells, but delivery to the lung epithelium remains a technical hurdle.
Meanwhile, advanced inhaled antibiotics (e.g., dry‑powder tobramycin) and personalized microbiome monitoring promise to reduce exacerbations further.
Key Takeaways
- The CFTR gene defect creates thick mucus that clogs the respiratory system.
- Chronic infections-especially Pseudomonas aeruginosa-drive inflammation and lung damage.
- Regular monitoring with sweat tests, genetic screening, and spirometry guides treatment.
- CFTR modulators have transformed prognosis, but airway clearance and nutrition remain vital.
- Emerging gene‑editing and mRNA therapies could eventually eliminate the root cause.
Frequently Asked Questions
What causes the thick mucus in cystic fibrosis lungs?
Mutations in the CFTR gene impair chloride transport, which reduces water on the airway surface. Without enough water, mucus becomes dense and sticky, making it hard to clear.
How is cystic fibrosis diagnosed?
The primary test is the sweat chloride test; values above 60mmol/L are diagnostic. Newborn screening adds a blood test for elevated IRT and CFTR DNA analysis. Confirmatory genetic sequencing identifies the specific mutation.
Can antibiotics cure lung infections in cystic fibrosis?
Antibiotics control bacterial growth but don’t eradicate biofilm‑protected colonies, especially Pseudomonas. Long‑term inhaled antibiotics reduce exacerbation frequency, while aggressive courses are used during acute flare‑ups.
What are CFTR modulators and who can use them?
CFTR modulators are drugs that improve the function of the defective CFTR protein. Eligibility depends on the specific mutation; newer triple‑combo therapy works for over 90% of patients with at least one ΔF508 allele.
How often should lung function be tested?
Routine spirometry is recommended every three months for children and adolescents, and at least twice a year for adults. More frequent testing is needed after a pulmonary exacerbation or when adjusting therapies.
Is gene therapy available for cystic fibrosis?
Clinical trials are ongoing, but no gene therapy has received regulatory approval yet. Early studies show promise, but challenges remain in delivering the gene to enough airway cells safely.
What lifestyle changes help manage lung disease?
Regular airway clearance (vest or manual techniques), staying up to date on vaccinations, avoiding tobacco smoke, maintaining good nutrition, and adhering to prescribed medications are key. Physical activity improves airway clearance and overall stamina.
Comments (20)
-
Andrea Dunn September 24, 2025
Don’t trust the pharma companies pushing these miracle drugs – they just want our tax dollars. 😊
-
Erin Johnson September 27, 2025
Ah, the marvels of modern medicine!
While the CFTR modulators sound like a sci‑fi fantasy, they genuinely lift FEV1 numbers for many patients.
Just remember, they’re not a silver bullet; airway clearance still reigns supreme.
So keep those vest sessions, and maybe the drama will stay on TV, not in the lungs.
-
Rica J September 29, 2025
Okay, so the CFTR gene is basically the gate‑keeper for chloride – when it’s broken, water stays away and mucus gets super sticky.
That’s why you see all the chronic infections, especially Pseudomonas – it loves a good biofilm party.
Also, don’t forget the daily chest physio – the vest isn’t just a fancy jacket, it actually shoves sputum out.
And lol, nutrition is key, those enzyme pills are life‑savrs!
-
Linda Stephenson October 1, 2025
It’s amazing how a single gene can set off a cascade that touches every part of daily life.
The routine of enzymes, inhalers, and vest sessions can feel endless, but each piece keeps the lungs from collapsing under that thick mucus.
Staying on top of nutrition and mental health makes a huge difference – you’re not just treating a disease, you’re supporting a whole person.
-
Sunthar Sinnathamby October 3, 2025
Listen up, champions – every breath you clear is a win against the invisible enemy.
Push that vest, hit the physio, and don’t let the fatigue win.
Remember, the stronger your airway clearance, the fewer hospital nights you’ll have.
Stay fierce, stay moving, and let the modulators do their magic while you keep the front line clean.
-
Catherine Mihaljevic October 6, 2025
So they tell us these “modulators” are the answer – sure, until the next pharma lobby pushes a pricier version.
Meanwhile, the “natural” approach gets ignored, as if brushing teeth is the only cure for all disease.
Typical narrative.
-
Michael AM October 8, 2025
Great rundown! Keep up the consistency with airway clearance and nutrition – the combo really does slow down lung decline.
Stay in touch with your care team, they’ll tweak therapies as needed.
-
Rakesh Manchanda October 10, 2025
One must appreciate the elegance of targeting the underlying molecular defect rather than merely managing symptoms.
The advent of triple‑combination therapy is a testament to scientific rigor and perseverance.
-
Erwin-Johannes Huber October 12, 2025
The balance between high‑tech modulators and good old‑fashioned physiotherapy is key.
Stay hopeful, stay diligent.
-
Tim Moore October 15, 2025
From a cultural perspective, the evolution of cystic fibrosis treatment reflects a broader shift toward personalized medicine, a paradigm that transcends national borders and underscores the universality of scientific collaboration.
It is incumbent upon clinicians worldwide to disseminate these advances equitably, ensuring that socioeconomic disparities do not curtail access to life‑altering therapies.
In this spirit, continued investment in translational research and global health initiatives remains paramount.
-
Erica Ardali October 17, 2025
The lungs, once pristine tapestries of oxygen, now bear the heavy cloaks of humanity’s hubris.
We fashion miracles in a bottle, yet cling to vest‑like relics of the past, as if the drama of mucus could ever be scripted without tragedy.
-
Justyne Walsh October 19, 2025
Ah yes, the noble American spirit battling thick mucus – nothing says patriotism like inhalers and chest physio.
It’s almost poetic how we glorify the struggle while ignoring the profiteers behind the curtain.
-
Callum Smyth October 21, 2025
Keep your head up, team!
Consistent airway clearance paired with the right meds makes a world of difference.
Remember, every sputum expectorated is a step toward fewer exacerbations.
Stay connected with your support network – you’re not alone in this journey. 😊
-
Xing yu Tao October 24, 2025
The contemporary management of cystic fibrosis epitomizes the convergence of molecular genetics and clinical praxis, a synthesis that warrants exhaustive contemplation.
Foremost, the delineation of CFTR genotypic variance furnishes a scaffold upon which targeted pharmacotherapy is erected, thereby transforming a historically fatal pathology into a chronic, manageable condition.
The introduction of potentiators such as ivacaftor inaugurated a paradigm shift, amplifying chloride channel activity in class III gating mutations and precipitating quantifiable increments in forced expiratory volume.
Subsequent combinatorial regimens, notably lumacaftor/ivacaftor, addressed the processing defect inherent to the ΔF508 allele, albeit with modest clinical gains tempered by hepatic considerations.
It is within the advent of triple‑combination therapy-elexacaftor/tezacaftor/ivacaftor-that we observe a commensurate elevation in FEV₁ surpassing fifteen percent, heralding a substantive amelioration of pulmonary function and quality of life.
Nevertheless, the exigency of relentless airway clearance persists; the vest, chest percussion, and high‑frequency oscillation collectively mitigate mucus stasis, a prerequisite for attenuating bacterial colonization.
Pathogenic biofilms, particularly those orchestrated by Pseudomonas aeruginosa, remain resilient adversaries, demanding an arsenal of inhaled antibiotics, including tobramycin and aztreonam, to suppress exacerbatory cascades.
Nutrition, often eclipsed in discourse, constitutes an indispensable axis of care, as pancreatic insufficiency necessitates exogenous enzyme supplementation and hypercaloric intake to sustain anabolic demands.
Moreover, psychosocial dimensions cannot be relegated to peripheral status; anxiety and depressive symptomatology are disproportionately prevalent, mandating integrated mental health services within multidisciplinary clinics.
The surveillance paradigm, leveraging quarterly spirometry and periodic high‑resolution computed tomography, affords clinicians a granular view of disease trajectory, enabling preemptive therapeutic modulation.
Prospective innovations, encompassing viral vector‑mediated gene delivery and CRISPR‑based genomic editing, aspire to rectify the underlying CFTR defect, yet logistical impediments and immunogenicity concerns temper optimism.
In parallel, emergent inhaled formulations and precision microbiome analytics promise to refine antimicrobial stewardship, curbing resistance development.
Ultimately, the synthesis of genotype‑guided pharmacology, diligent physiotherapy, nutritional optimization, and psychosocial support coalesces into a comprehensive care model, substantially extending survival and enhancing life quality for individuals afflicted with cystic fibrosis.
-
Adam Stewart October 26, 2025
Just wanted to say I’m here, reading quietly, and I appreciate the thoroughness.
-
Selena Justin October 28, 2025
Thank you for the comprehensive overview. It is crucial to maintain both pharmacologic and physiotherapeutic interventions to optimize outcomes for patients.
-
Bernard Lingcod October 30, 2025
Interesting how the sweat test still remains the gold standard despite advances in genetic screening. It underscores the importance of multimodal diagnostics.
-
Raghav Suri November 2, 2025
Yo, keep the vest on and the meds tight. The lungs don’t wait for anyone.
-
Freddy Torres November 4, 2025
Blend science with soul – keep clearing, keep thriving.
-
Andrew McKinnon November 6, 2025
Look, the airway clearance regimen is just another piece of the care protocol stack – treat the mucus load, monitor FEV1 metrics, and iterate the therapeutic algorithm. Got it?
